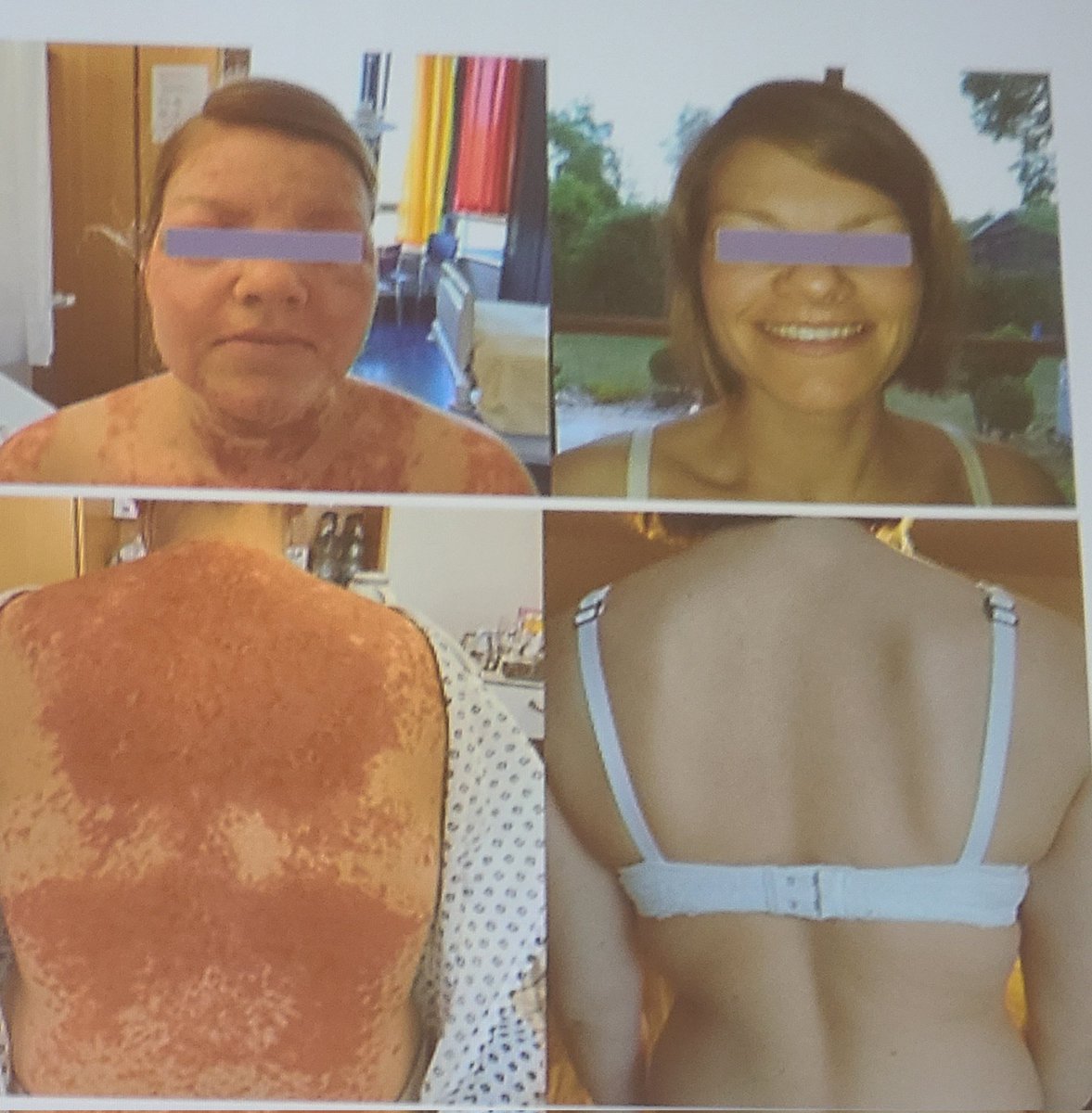
FDA has granted Fast Track designation to IMPT-514, a bispecific CD19/CD20-targeting chimeric antigen receptor (CAR) T-cell therapy for the treatment of active refractory lupus nephritis (LN) and systemic lupus erythematosus (SLE).
FDA has granted Fast Track designation to IMPT-514, a bispecific CD19/CD20-targeting chimeric antigen receptor (CAR) T-cell therapy for the treatment of active refractory lupus nephritis (LN) and systemic lupus erythematosus (SLE).
17
39
264
127K
40
Download Image